

The Impact of ISO 11607 on Packaging for Sterilized Medical Devices
ISO 11607 is a critical standard that outlines the requirements for packaging materials and systems used to package sterilized medical...


Understanding Biocompatibility: A Straightforward Guide to ISO 10993-1 Standards
Understanding the intricacies of ISO 10993-1 standards is crucial for the development and approval of medical devices. These standards...


Decoding the ISO 10993 Series: Evaluating Biocompatibility in Medical Devices
The ISO 10993 series sets the gold standard for evaluating the biocompatibility of medical devices, ensuring that they are safe for their...


Innovation with Compliance: Merging ISO 62304 Software Life Cycle into Medical Device Creation
The convergence of innovative software development and stringent regulatory compliance is a hallmark of modern medical device creation....


Achieving Clinical Excellence: The Role of ISO 14155 in Medical Device Trials
The International Organization for Standardization (ISO) 14155 standard plays a pivotal role in medical device clinical trials, setting...


Navigating ISO 14971: Risk Management for Medical Devices Explained
ISO 14971 serves as a cornerstone for risk management in the development and lifecycle of medical devices. It provides a thorough...

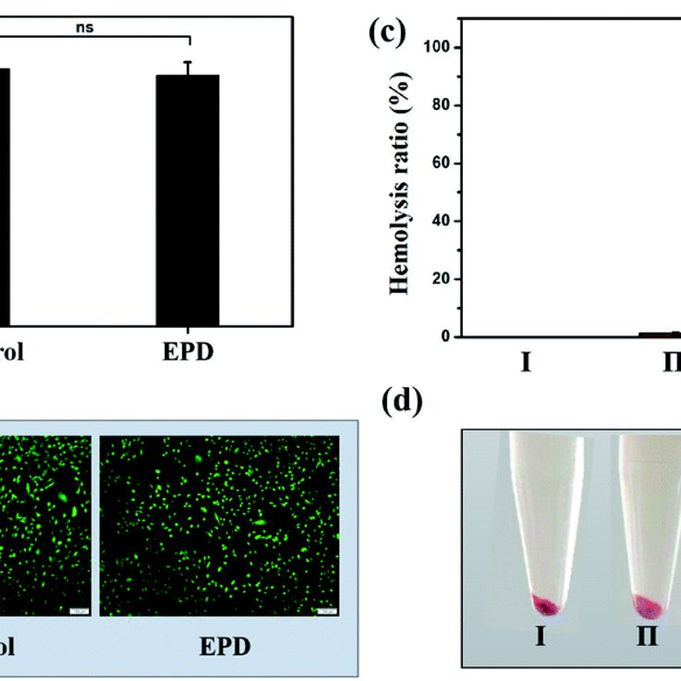
ISO 10993-1 and Biocompatibility: Safeguarding Patient Health
In the realm of medical device development, ensuring the safety and compatibility of materials with the human body is paramount. ISO...


Implementing ISO 15223-1: Decoding Symbols and Labeling Requirements for Medical Devices
In the ever-evolving field of medical device manufacturing, adhering to international standards is crucial for ensuring product safety...


Biocompatibility Standards Under ISO 10993-1: Ensuring Patient Safety Through Material Assessment
The article 'Biocompatibility Standards Under ISO 10993-1: Ensuring Patient Safety Through Material Assessment' provides an in-depth...


Unlocking the Secrets to Medical Device Success: An Analysis of the Top 5 ISO Standards
The medical device industry is governed by stringent regulations to ensure the safety and efficacy of products. Among these regulations,...


Deciphering ISO 11608: Ensuring Safety in Needle-based Injection Systems for Medical Devices
ISO 11608 is a critical standard for ensuring the safety and efficacy of needle-based injection systems used in medical devices. This...


Ensuring Quality and Compliance: How Vital ISO Standards Shape Medical Device Innovation
The medical device industry is a critical component of the healthcare sector, where innovation must be matched with stringent quality and...


Deciphering ISO 14971: A Deep Dive into Risk Management for Medical Device Manufacturers
ISO 14971 serves as a critical framework for risk management in the medical device industry, providing comprehensive guidelines for...


ISO 60601-1 and Patient Safety: Ensuring Medical Device Reliability
ISO 60601-1 is a critical standard for the safety and reliability of medical devices. It sets forth the necessary guidelines to ensure...


Navigating through ISO Standards: A Closer Look at Medical Device Compliance
The landscape of medical device manufacturing is complex and regulated, with ISO standards playing a pivotal role in ensuring the safety,...

The Impact of Risk Management on Design Control: Breaking Down ISO 14971 for Medical Devices
The article 'The Impact of Risk Management on Design Control: Breaking Down ISO 14971 for Medical Devices' offers a comprehensive...


Exploring ISO 14971: Managing Risks in Medical Device Development
ISO 14971 is a critical standard for medical device manufacturers, outlining a comprehensive risk management process designed to ensure...


Understanding the ISO 13485: Navigating Medical Device Quality Management Systems
The ISO 13485 standard is a globally recognized framework for establishing and maintaining a quality management system (QMS) tailored to...


Understanding ISO 13485: The Cornerstone of Medical Device Quality Management Systems
ISO 13485 is a globally recognized standard that specifies requirements for a quality management system (QMS) specifically designed for...


Understanding the AAMI TIR45: Guidance on Agile Practices in Medical Device Development
The AAMI TIR45 is a pivotal document that provides guidance on the use of Agile practices in the development of medical devices, helping...


The Impact of IEC 60601 on Medical Device Electrical Safety Standards
The International Electrotechnical Commission's IEC 60601 standard is a cornerstone in ensuring the safety and effectiveness of medical...


Deciphering ISO 14971: Mastering Risk Management in Medical Device Development
ISO 14971 is a pivotal standard for medical device manufacturers, providing a comprehensive framework for managing risks throughout the...

Elevating Performance: Understanding ISO 60601 Series Standards for Medical Equipment
The ISO 60601 series of standards represents a cornerstone in ensuring the safety and performance of medical electrical equipment. As...

Navigating ISO 14971: Risk Management in Medical Device Manufacturing
ISO 14971 is a crucial standard for risk management in the manufacturing of medical devices. It provides a comprehensive framework for...



